Rising environmental concerns are driving many people to look for peat-free Ericaceous compost to grow their acid-loving plants.
But, how do you make peat-free ericaceous compost?
The best substitute for peat in making ericaceous compost is sulfur or coco coir. Other biodegradable organic materials such as pine needles, pine bark, oak leaves, coffee grounds, vinegar, citric juice, or even fish tank water are, however, not effective in creating any significant and long-term drop in the soil pH.
If you want to know why these two are alternatives to peat and why the others don’t work, read on.
1. What is Ericaceous Compost?
“Ericaceous” refers to Ericaceae, or the heather family, consisting of over 1900 species ranging from alpine herbs to large tropical trees.
These plants prefer to grow in soil with pH 4.5 – 5.5, which is much more acidic than the pH preference of most plants in the range of pH 6 – 7. Because of this, they are also known as “acid-loving plants”.
The growing medium suitable for growing such acid-loving plants is thus called “ericaceous compost” which typically has a 4.5 – 5.5 pH.
There is no single, specific recipe for making ericaceous compost. Ericaceous compost is produced by simply mixing acid-forming substances, which can be minerals or organic matter, into the soil to achieve a low pH for acid-loving plants.
2. Ericaceous Compost vs. Regular Compost
Ericaceous compost is prepared on the site where the acid-loving plant is and requires no decomposition prior to use, unlike regular compost which needs to decompose and mature first for several months before use.
Because of such a difference, the pH of regular compost returns to almost neutral at the end of the decomposition process.
3. Why Do We Need Ericaceous Compost?
Acid-loving plants, including plants from the heather family, need to be grown in soil with a 4.5-5.5 pH, which is much more acidic than what most plants prefer.
This is because acid-loving plants have a much higher requirement of certain minerals such as Manganese (Mn) and Iron (Fe) which are most available in highly acidic environments.
Most plants, however, do not require much Iron or Manganese and can do with the small amounts that their roots can take in.
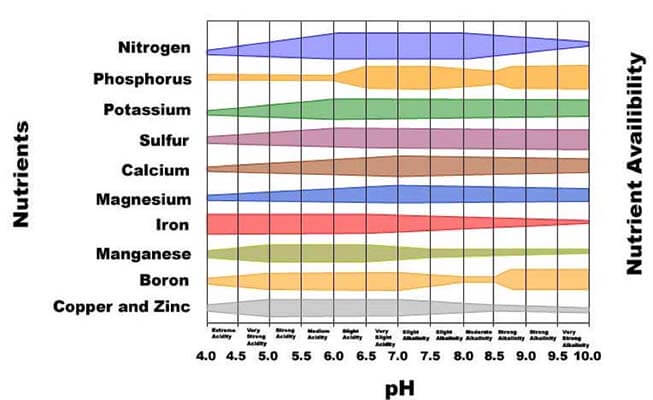
Iron and manganese are needed for photosynthesis and chlorophyll production, and a deficiency in these would cause chlorosis or yellowing of leaves.
Ericaceous compost can thus lower the soil pH, making such minerals become more soluble and available to the plant for immediate uptake.
3.Best Peat-Free Ways to Acidify Soil Naturally
3.1 Coco Coir for pots
For potted plants, coco coir, or coconut shell fibers, is the best natural alternative to peat moss in acidifying the soil and improving nutrient uptake for acid-loving plants.
This is shown by an experiment that compares the use of 20% peat as opposed to 20% coco coir in amending the pH of a bark growing medium for 16 weeks (Scagel, 2003).
The results show that although coco coir does not lower the pH of the growing medium as much as peat does, a coir-amended medium actually provides the same availability of Magnesium (Mg), Iron (Fe), and Boron (B) minerals and even better availability of nutrient including Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Sulfur (S) for immediate uptake than a peat-amended medium.
The coir-amended medium resulted in slightly weaker roots but stronger stems, compared with the peat-amended medium.
To make ericaceous compost using coco coir, mix 1 part of coconut coir with 1 part of garden soil or regular compost.
3.2 Sulfur for garden beds
Elemental sulfur is the most effective in lowering soil pH for acidifying a large quantity of soil, for example, for use outdoors in garden beds. Using coco coir would not be cost-effective for a large area as it would require a lot to change just one-tenth of a pH point.
For application, till 2 pounds of sulfur in the top 12 inches of soil per 100 square feet. Reapply at least one more time a year later. And check the soil pH before reapplication.
Changing the soil pH is a slow process because it relies on soil bacteria to convert the sulfur in the soil (or compost) into sulfuric acid (H2SO4), and the process can take up to a year.
The best time to apply sulfur is in the spring and summer when soil temperatures are above 55°F (13°C), and when soil bacteria are more active. This can cut down the time needed by more than half.
Other forms of sulfur, like iron sulfate (FeSO4) and aluminum sulfate (Al2(SO4)3), can produce faster results, lowering the pH within a day. But this may shock the existing plants (e.g. lawn) and the microorganisms in the soil. It would also require using up to five times more of the chemicals than elemental sulfur as it may easily leach away. And, the use of such chemicals is not compatible with organic gardening.
4. What Doesn’t Work?
There are many suggestions for using different organic matter to lower soil pH, but most biodegradable organic material does not work.
4.1 Pine Bark, Pine Needles, Sawdust
One of the popular garden myths for acidifying the soil is to compost or mulch with pine bark, pine needles or even sawdust.
It is true that fresh green pine needles on the tree have a pH of 3.2 to 3.8. But their pH drops when they mature and drop from the tree. When they decompose in the soil and turn brown, their pH quickly returns to neutral within a few days.
Pine bark, pine dust, and other wood chips have a similar problem: it would require dumpster loads to have any measurable change, and the pH returns to neutral quickly when they decompose.
4.2 Deciduous Leaves
Another garden myth is that leaves from deciduous trees such as oak, maples, and beech, can acidify soils.
Fresh oak leaves have a pH of 4.5 t0 4.7. But, similar to the problem with pine needles, it would require a lot of oak leaves to make a significant change and the effect is not long-lasting.
4.3 Vinegar
Using vinegar (which typically has a pH of 2.4) to acidify soil is instant, within hours of application.
Do not put vinegar directly into the soil, as it is too acidic and can shock the existing plants and soil microbes. Instead, dilute one cup of vinegar with a gallon of water, and use the solution to water the soil.
The acidifying effect using vinegar, however, is not permanent as it breaks down quickly in soil. Moreover, the acidity will be further reduced quickly by rainfall and subsequent watering.
It would require frequent applications and you would need to check the soil pH every few days.
4.4 Lemon/Lime Juice
Using concentrated lemon and lime juice to acidify the soil is also an ineffective and short-term method. Although they have low pH levels and can be used diluted in water, their acidity is quickly reduced by rainfall and watering.
4.5 Coffee Grounds
Using coffee grounds to acidify soil is ineffective and unreliable because only fresh, unused coffee grounds are acidic. Used coffee grounds have a near-neutral pH since most of the acids have gone into the drink.
Also, the acidifying effect of fresh coffee grounds is short-lived since the acid is water-soluble and will be washed out of the soil after watering a couple of times.
You should also know that the caffeine in coffee can suppress seed germination and stunt plant growth.
4.6 Fish Tank Water
The water in poorly maintained fish tanks which isn’t changed often typically is slightly acidic due to the organic waste produced by the fish. However, it would require a lot of fish tank water to create a significant and long-term change in soil pH.
Another problem with fish tank water is its foul smell which is not optimal for indoor plants. And, such water can carry pathogens that would not be optimal for use in the vegetable garden.
5. Does Ericaceous Compost Lose Acidity Over time?
Yes. All kinds of ericaceous compost lose their acidity over time, becoming neutral as the compost matures in six months up to a year.
Using ericaceous compost to acidify soil would thus require repeated applications once every six months or every year, depending on the ingredients in the compost.
Compost that contains sulfur is the most stable and can remain acidic for up to two years.
6. Does Ericaceous Compost Work with Pots?
Ericaceous compost works well with acid-loving plants in pots. In fact, it may actually be easier to grow such plants in outdoor containers in places where the soil is not acidic enough or even alkaline.
This is because amending soil pH in large areas such as garden beds requires multiple applications of ericaceous compost once every 6 months and frequent checks to monitor the soil pH from time to time.
Fill the pot or container with a mix of equal amounts of ericaceous compost and coarse sand.
7. Is Multi-Purpose Compost Ericaceous?
Multi-purpose compost is not ericaceous and is only slightly acidic to pH neutral.
Multi-purpose compost can improve water retention and the aeration of the soil. But it is not acidic enough for ericaceous plants.
8. Can I Use Ericaceous Compost On All Plants?
It is not recommended to use ericaceous compost (with pH 4.5-5.5) for all plants because different plants have different pH preferences and tolerances.
Most plants require only a slightly acidic growing environment (pH 6-7) and will have difficulty in taking up nutrients in a highly acidic medium.
Some plants (e.g. lavender, thyme) even prefer slightly alkaline soil, and thus will struggle to grow in soil amended with ericaceous compost.
9. How to Use Ericaceous Compost In Gardens
You can use ericaceous compost in the garden in different ways, depending on the soil type in your garden.
If your garden soil is neutral or slightly acidic, you can work the compost into the topsoil or lay it on the surface as mulch, and the organic organisms will eventually work it into the soil.
Or, you can make raised beds for your acid-loving plants. Fill the beds with a mixture of equal amounts of garden soil, ericaceous compost, and one part of coarse sand.
If done right, raised beds can allow you to grow both ericaceous and alkaline-loving plants in the same garden.
Conclusion
The best peat-free ericaceous compost uses sulfur or coconut coir to acidify the soil.
Other organic materials such as pine needles and bark, wood chips, sawdust, coffee grounds, oak leaves, citrus fruit juice, vinegar, fish tank water, however, are not effective in maintaining significant and long-term changes to the soil pH.
Happy growing!
Related
Top 41 Acid-Loving Plants (A to Z list)
Peat vs Humus: Know the Differences & Uses
Potting Soil vs. Compost: Know the Differences & Uses
References
BBC Gardeners’ World Magazine. (2021). Ericaceous compost.
Oregon state university (2017). Myth vs. reality: What’s the truth behind some common gardening practices?
Winkler, M. G. & DeWitt, C. B. (2009). Environmental Impacts of Peat Mining in the United States: Documentation for Wetland Conservation | Environmental Conservation. Cambridge Core.
Layman, K., Dunn, B. & Arnall, B. (2018). Identifying and Correcting Iron Deficiencies in Ornamentals – Oklahoma State University. Oklahoma State University.
Longstroth, M. (n.d.). Lowering the Soil pH with Sulfur. Michigan State University Extension.
Morrissey, J. & Guerinot, M. L. (2010). Iron uptake and transport in plants: The good, the bad, and the ionome. Chem Rev. 2009 Oct; 109(10): 4553–4567.
Scagel, C. F. (2003). Growth and Nutrient Use of Ericaceous Plants Grown in Media Amended with Sphagnum Moss Peat or Coir Dust. American Society for Horticultural Science.
- Top 6 Drip Irrigation Systems for Raised Beds (2025) - January 31, 2025
- Top 10 Orchid Fertilizers: A Comprehensive Review (2025) - January 16, 2025
- Top 6 Slow-Release Fertilizers for Houseplants & Veggies (2025) - January 15, 2025

